When someone is taking clozapine for treatment-resistant schizophrenia, their smoking habit isn’t just a personal choice-it’s a medical factor that can make the difference between effective treatment and dangerous side effects. If you smoke, your body breaks down clozapine faster. If you quit, it slows down. And if you don’t adjust the dose accordingly, you could end up with too little drug in your system-or too much, leading to serious harm.
Why Smoking Changes Clozapine Levels
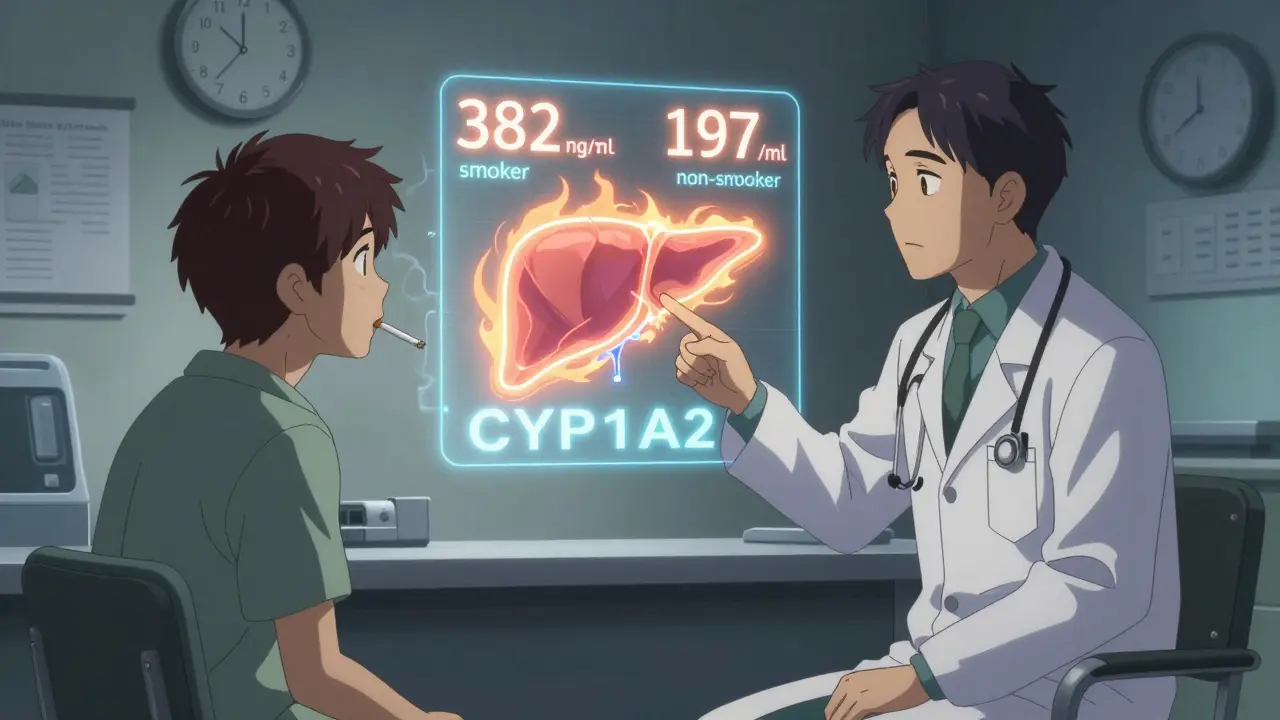
Clozapine is metabolized almost entirely by one liver enzyme: CYP1A2. That’s unusual. Most drugs are broken down by several pathways, so if one gets blocked or boosted, others can pick up the slack. But clozapine? It’s all on CYP1A2. And cigarette smoke? It’s one of the strongest known activators of this enzyme. The chemicals in tobacco smoke-especially polycyclic aromatic hydrocarbons (PAHs)-tell your liver to produce more CYP1A2. Within 24 to 48 hours of starting to smoke, this enzyme ramps up. After about 20 cigarettes a day, you hit maximum induction. Studies show that smokers need, on average, 50% to 100% more clozapine than non-smokers just to reach the same blood levels. One major study found smokers required 382 mg per day, while non-smokers managed with 197 mg. That’s more than double. Why does this matter? Clozapine has a narrow therapeutic window: 350 to 500 ng/mL. Below that, symptoms of schizophrenia return. Above it, you risk seizures, heart inflammation, or even life-threatening drops in white blood cells. Smoking pushes levels toward the low end. Quitting? That’s when things get dangerous.What Happens When You Quit Smoking
If you’re on clozapine and you stop smoking, your body doesn’t adjust right away. The CYP1A2 enzyme doesn’t vanish overnight. It takes about 38.6 hours to drop by half. After two days, activity falls by 20%. By day seven, it’s down 36%. But your clozapine dose? Still the same. That’s a recipe for toxicity. Research shows that within two weeks of quitting, clozapine blood levels rise by an average of 29.3%. But averages don’t tell the whole story. Some patients see a 10% increase. Others jump over 200%. One case report described a 45-year-old man who went from feeling fine to delirious and in intensive care after just 10 days of not smoking. His clozapine level hit 1,200 ng/mL-more than double the safe upper limit. Clinicians now agree: if a patient quits smoking, you need to reduce their clozapine dose immediately. The standard recommendation is a 25% to 30% cut right away. Then, check blood levels weekly for the next two weeks. Don’t wait for symptoms. Don’t assume “they’ll tell us if they feel off.” Many patients don’t notice subtle changes until it’s too late.Vaping? It’s Not Safer
Many patients switch from cigarettes to vaping, thinking it’s harmless. But vaping isn’t a neutral substitute. While it lacks the combustion byproducts that strongly induce CYP1A2, some vape liquids contain aldehydes and carbonyls that can still affect liver enzymes. The result? Unpredictable. Some patients who switch to vaping see their clozapine levels rise-similar to quitting smoking. Others see no change. A few even report lower levels, possibly because vaping doesn’t fully suppress enzyme activity the way cigarettes do. There’s no clear pattern. That’s why guidelines now say: if you change from smoking to vaping, treat it like a smoking cessation. Monitor clozapine levels closely for at least two weeks.
Genetics Don’t Save You
You might have heard about genetic testing for drug metabolism. Some people carry a variant in the CYP1A2 gene (called *1F) that makes them more sensitive to enzyme inducers. You’d think that would matter here. But research says otherwise. A 2003 study of 80 patients found no link between this genetic variant and the dose needed. Whether you had the high-inducibility gene or not, if you smoked, you needed more clozapine. If you didn’t smoke, you needed less. Behavior-smoking or not-overruled genetics every time. This is important. It means you can’t rely on a DNA test to guide your dose. You need to know whether the person smokes, how much, and whether they’ve changed habits recently.Other Drugs, Less Drama
CYP1A2 metabolizes other medications too-like olanzapine and caffeine. But none of them are as sensitive as clozapine. Olanzapine, another antipsychotic, sees about a 30% drop in levels in smokers. But because it’s broken down by multiple enzymes, dose adjustments are usually modest. Methadone? Smokers need 20-30% more. Still, nowhere near clozapine’s 50-100%. Clozapine’s unique vulnerability comes from its total reliance on CYP1A2. It’s the only psychiatric drug where this single enzyme handles nearly all the work. That’s why this interaction is so critical-and why it’s often missed.
What Clinicians Need to Do
Every patient on clozapine should be asked about smoking at every visit. Not just at the start. Not just when they’re admitted. Every time. Because habits change. Here’s what works:- For smokers: Start at standard doses but expect to go 50-100% higher. Track the concentration-to-dose ratio (C/D). Smokers usually have ratios under 0.8 (ng/mL per mg/day). Non-smokers are around 1.5-2.0.
- For those quitting: Reduce dose by 25-30% on day one. Check blood levels at day 7 and day 14. Adjust further based on results.
- For vapers: Treat like a smoker who quit. Monitor levels for two weeks.
- For heavy coffee drinkers: Caffeine competes for CYP1A2. If someone drinks 5+ cups of coffee daily, they may need slightly higher clozapine doses-even if they don’t smoke.
Real Consequences, Real Solutions
Improper management of this interaction isn’t just a theoretical risk. It leads to real hospitalizations. One study estimated that 15-20% of clozapine-related admissions are preventable if smoking status is tracked and doses adjusted. That’s about $12,500 per avoidable stay. On the flip side, when done right, outcomes improve dramatically. One patient, after quitting smoking, had weekly blood tests. Her dose was lowered slowly from 450 mg to 250 mg over 10 days. She stayed symptom-free. No side effects. No panic. The tools are there. The science is clear. The guidelines are well-established. What’s missing is routine practice.What You Should Ask
If you’re on clozapine:- Have you changed your smoking habits in the last month?
- Have you cut down, quit, or switched to vaping?
- Have you had a blood test for clozapine levels since your last change?
- Is smoking status documented in the chart? Updated?
- When was the last TDM result? Is it within therapeutic range?
- Have you adjusted the dose after a change in smoking behavior?