When a patient walks out of the clinic with a new prescription, they’re not just getting a pill-they’re getting a promise. A promise that the medicine will work. That it won’t break their budget. That they can actually take it every day without fear or confusion. But too often, that promise gets broken-not because the drug doesn’t work, but because the patient doesn’t understand why they’re getting a different-looking pill than what they saw on TV.
The truth is, generic medications are just as safe and effective as brand-name drugs. The FDA requires them to meet the same strict standards: same active ingredient, same strength, same way of being taken. They must also prove they work the same way in the body. That’s called bioequivalence. It’s not a guess. It’s science. And yet, nearly 1 in 3 patients still believe generics are inferior. Why? Because no one took the time to explain.
Why Providers Are the Key to Generic Adoption
Doctors and pharmacists don’t just write prescriptions-they shape trust. Studies show that when patients hear from their provider that a generic is just as good, they’re far more likely to take it. In fact, research published in PMC found that patient trust in their doctor often overrides their own doubts about generics. That’s powerful. It means your words matter more than any ad, any label, or any price tag.
But here’s the problem: most visits are short. The average primary care visit lasts 13 to 16 minutes. In that time, you’re diagnosing, explaining treatment options, answering questions about side effects, and maybe even addressing a patient’s anxiety about their condition. Talking about generic substitution doesn’t always make the list. But it should.
Why? Because cost directly affects whether someone takes their medicine. The Association for Accessible Medicines found that new patients abandon brand-name drugs at 266% higher rates than generics. Why? Copays. Ninety percent of generic copays are under $20. Only 39% of brand-name copays are. That’s not a small difference-it’s the difference between taking your blood pressure pill or skipping it because you can’t afford it.
The Science Behind Generics (And Why It Doesn’t Scare Patients)
Some patients worry: “If it looks different, is it different?” They’re not wrong to ask. Generics can have different colors, shapes, or fillers. But those are inactive ingredients-things that don’t affect how the drug works. They’re like the wrapper on a candy bar. The chocolate inside is the same.
The FDA’s approval process is rigorous. To get approved, a generic must match the brand in active ingredient, dosage, and how quickly it enters the bloodstream. The acceptable range for bioequivalence is tight: between 80% and 125% of the brand’s performance. That’s not a wide margin. That’s precision. And it’s why generics are used in over 90% of all prescriptions filled in the U.S.-but only 23% of total drug spending.
Still, patients hear stories. “My cousin took a generic for cholesterol and felt weird.” Or, “I switched and my headache got worse.” These anecdotes stick. But they’re not data. They’re noise. And as a provider, you’re the one who can cut through it.
When Generics Aren’t the Best Choice
Not every drug is interchangeable. Some medications have a narrow therapeutic index-meaning the difference between a helpful dose and a harmful one is tiny. Drugs like warfarin, levothyroxine, and certain seizure medications fall into this category. For these, switching between brands and generics can sometimes cause problems, even if the science says they’re equivalent.
The American Academy of Family Physicians (AAFP) rightly opposes mandatory generic substitution for these cases. But that doesn’t mean you shouldn’t talk about it. It means you need to be specific. Say: “This medicine needs to be very consistent. Let’s stick with what’s working unless we have a good reason to change.”
And here’s the nuance: even for these drugs, many patients do fine switching-especially if they’re monitored closely. The key isn’t to avoid generics entirely. It’s to make informed, individualized choices. That’s advocacy.
How to Talk About Generics Without Losing Time
You don’t need a 10-minute lecture. You need a clear, confident sentence.
- “This generic version has the same active ingredient as the brand, and the FDA says it works just as well.”
- “The copay is under $20-most brand versions cost over $50. This means you’re more likely to be able to take it every day.”
- “The shape or color changed because it’s made by a different company. The medicine inside is the same.”
- “If you’ve had a bad experience with a generic before, let’s talk about it. Maybe we can find one that works better for you.”
Pharmacists are your partners here. Many now offer counseling when a patient picks up a new generic. But if you don’t set the tone first, patients come in with doubt already planted. That’s why your voice matters before the pill is even dispensed.
One simple trick: mention the cost difference during the visit. Say, “This generic will save you about $40 a month. That’s enough for groceries or gas. Would you like to try it?” That turns a clinical decision into a personal one. And patients respond to personal relevance.
What’s Changing in the Generic Market
Generics aren’t always cheap anymore. In 2023, the American Society of Health-System Pharmacists warned that some essential generics-like insulin, antibiotics, or heart medications-have seen sudden price spikes. Some have doubled or tripled in cost. That’s a crisis.
So now, advocacy isn’t just about switching to generics. It’s also about knowing when they’re no longer affordable. You need to check your pharmacy’s formulary before prescribing. Ask: “Is this generic still priced fairly?” If not, you may need to reach out to patient assistance programs, consider alternatives, or even advocate for policy changes.
And don’t ignore the administrative barriers. Prior authorization for generics still exists in some plans. Studies show it delays treatment by over two days. That’s two days where a patient might get sicker. The AAFP recommends eliminating prior auth for generics. You can help by pushing back when your system requires it.
Real Impact: What Happens When Providers Advocate
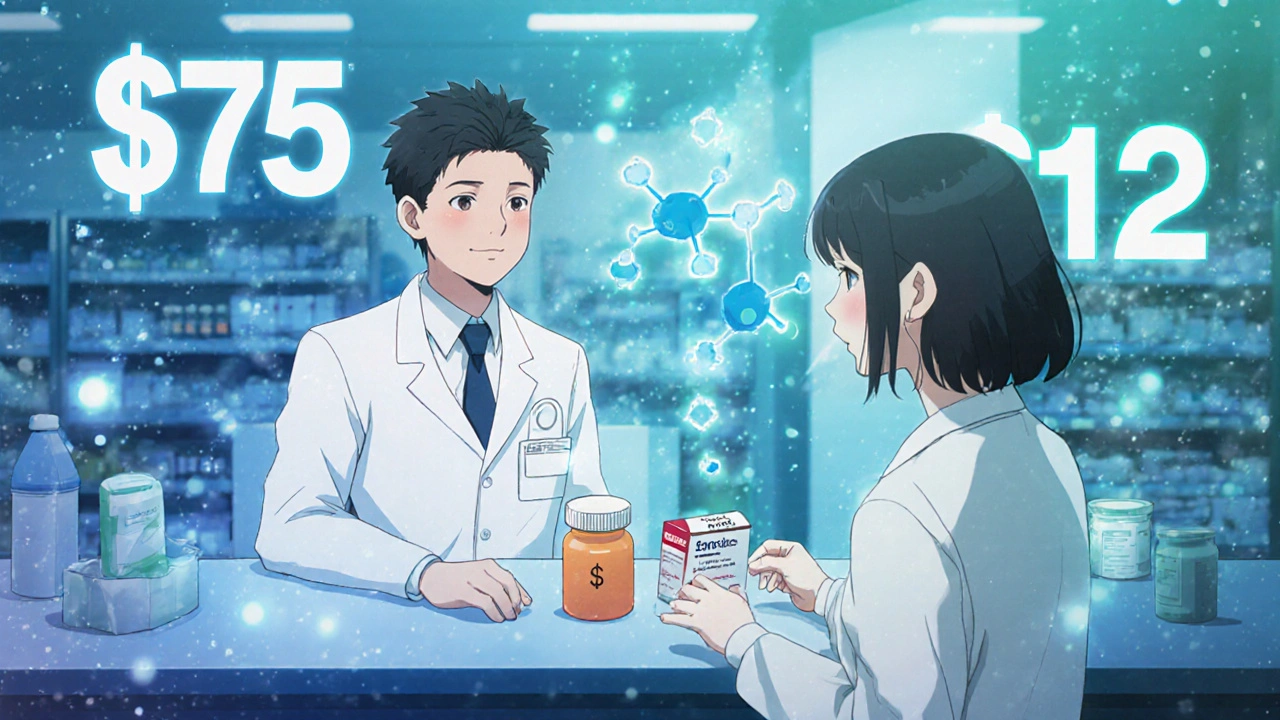
Think about a patient with high blood pressure. They’re on a brand-name drug with a $75 monthly copay. They skip doses. Their pressure stays high. They end up in the ER. Now imagine the same patient gets the same drug as a generic-with a $12 copay. They take it daily. Their pressure drops. They avoid hospitalization. That’s not just cost savings. That’s better health.
It’s not magic. It’s simple: when providers explain, patients believe. When providers care about cost, patients feel seen. When providers take five extra seconds to say, “This works the same and costs less,” they’re not just prescribing medicine-they’re removing a barrier to healing.
And in a system where 4 in 10 Americans skip medication because of cost, that’s not optional. It’s essential.
What’s Next? Making Advocacy Routine
Electronic health records are starting to show real-time pricing at the point of prescribing. Soon, you’ll see a pop-up: “This generic saves $52/month.” That’s helpful. But it’s not enough. You still need to say it aloud.
Start small. Pick one chronic condition-diabetes, asthma, high cholesterol-and commit to always offering the generic first, unless there’s a clear clinical reason not to. Then, make sure you explain why. Track what happens. Do more patients refill? Do fewer call in with complaints? That’s your data.
Advocacy isn’t about pushing generics. It’s about empowering patients to make the best choice for their health and their wallet. And that’s something every provider can do-every day, in every visit.