When you pick up a generic pill at the pharmacy, you might assume it’s just a cheaper copy. But here’s the truth: generic drugs don’t just mimic brand-name drugs-they must match them exactly in safety, strength, and performance. The U.S. Food and Drug Administration (FDA) doesn’t approve generics because they’re affordable. They approve them because they’ve proven to be identical in how they work inside your body.
What Makes a Generic Drug Approved?
The FDA doesn’t require generic manufacturers to repeat the years-long clinical trials that brand-name drug companies do. Instead, they use a shortcut called the Abbreviated New Drug Application (ANDA). But don’t let the word “abbreviated” fool you. This process is one of the most technically demanding in pharmaceutical regulation. To get approved, a generic drug must have the same active ingredient, dosage form, strength, and route of administration as the original brand-name drug. That’s not just a guideline-it’s a legal requirement. If the brand-name drug is a 20 mg tablet taken by mouth, the generic must be exactly that. No more, no less. But here’s what most people don’t realize: the inactive ingredients can be different. Fillers, dyes, and binders don’t affect how the drug works, so they can vary. That’s why some people report feeling different on a generic version-it’s not the active drug changing, it’s the way the tablet dissolves or how your stomach reacts to a new coating.Bioequivalence: The Core of Generic Approval
The real test for a generic drug isn’t how it looks or what’s in the bottle. It’s how your body absorbs it. That’s where bioequivalence comes in. The FDA requires generic drugs to be absorbed into your bloodstream at the same rate and to the same extent as the brand-name version. The acceptable range? Between 80% and 125% of the brand-name drug’s absorption. This isn’t arbitrary. It’s based on decades of clinical data showing that within this range, there’s no meaningful difference in how the drug performs in patients. For example, if a brand-name drug reaches a peak blood concentration of 100 ng/mL, the generic must hit between 80 and 125 ng/mL. This is tested in small studies with healthy volunteers-usually 24 to 36 people-who take both the brand and generic versions in a crossover design. Blood samples are taken over time to measure key metrics like Cmax (highest concentration) and AUC (total exposure over time). For simple pills, this is straightforward. But for complex products like inhalers, patches, or extended-release tablets, the testing gets much harder. A drug like Ritalin LA, which releases medication slowly over hours, requires multiple blood draws at specific time windows to prove it releases at the same pace as the brand. The FDA’s Question-Based Review (QbR) system digs into every detail of the manufacturing process to make sure these release patterns are consistent.Manufacturing Standards: No Compromises
A generic drug isn’t approved just because it works in a lab. It has to be made the right way, every single time. All generic manufacturers must follow Current Good Manufacturing Practices (cGMP), which are strict rules outlined in 21 CFR Parts 210 and 211. These cover everything from the cleanliness of the factory floor to how raw materials are stored and how batches are tested. The FDA inspects about 1,200 manufacturing sites each year before approving a generic. If they find a single major flaw-like inconsistent tablet hardness or contaminated equipment-the application gets a Complete Response Letter. That means the company has to fix the issue and resubmit. In 2021, Hetero Labs had its generic version of Jardiance rejected because tablet hardness varied across batches. That’s not a small detail. Uneven hardness can mean some pills dissolve too fast, others too slow. That’s dangerous. Successful companies don’t wait for inspections. They invest in quality systems from day one. Teva Pharmaceuticals’ regulatory team says new applicants are often shocked by how much data the FDA expects: at least three full commercial-scale batches, with full documentation of every step. One mistake in documentation can delay approval by months.
Narrow Therapeutic Index Drugs: Tighter Rules
Not all drugs are created equal. Some, like warfarin (a blood thinner), levothyroxine (for thyroid conditions), or phenytoin (for seizures), have what’s called a narrow therapeutic index. That means the difference between a safe dose and a toxic one is very small. For these drugs, the FDA doesn’t accept the standard 80%-125% range. Instead, they tighten the rules. For levothyroxine generics, the approved range is now 95%-105%. That’s a much narrower window-because even a 5% difference in absorption can cause symptoms like palpitations or fatigue. The FDA’s 2019 guidance on these drugs requires extra testing and stricter controls. Manufacturers must prove not just bioequivalence, but consistency across multiple batches. This is why you’ll often hear doctors say, “Stick with the same brand or generic.” It’s not because generics are unsafe-it’s because switching between different generics can cause small but meaningful changes in blood levels.Why Do Some Generics Get Rejected?
You might think that since generics don’t need full clinical trials, they’re easier to get approved. The opposite is true. Only about 10% of generic applications get approved on the first try. Compare that to 90% of new drug applications. Why? Because the bar for generics is just as high-it’s just a different kind of high. Complex products are the biggest hurdle. Inhalers, topical creams, and injectable suspensions are incredibly hard to replicate. The FDA approved only 3 out of 27 generic versions of the EpiPen between 2015 and 2020. Why? Because it’s not just about the drug-it’s about the device. The needle depth, the force of the injection, the stability of the epinephrine in the cartridge-all of it has to match perfectly. Even for simple pills, incomplete submissions cause delays. One company submitted an ANDA with missing stability data for a 12-month period. The FDA rejected it. The company had to redo the testing, which added 18 months to the timeline.How Long Does It Take? How Much Does It Cost?
Bringing a generic drug to market takes time-and money. The average cost? Around $1.3 million. That’s a fraction of the $2.6 billion it costs to develop a new brand-name drug. But for complex generics, costs can jump to $25 million. The timeline? About 32.7 months from submission to approval. For conventional pills, it’s closer to 28.5 months. For complex products, it’s nearly 47 months. The FDA has review timelines built into the Generic Drug User Fee Amendments (GDUFA). Standard applications get a first review within 10 months. Priority ones? 8 months. But if the application is incomplete or flawed, the clock stops until the company fixes it.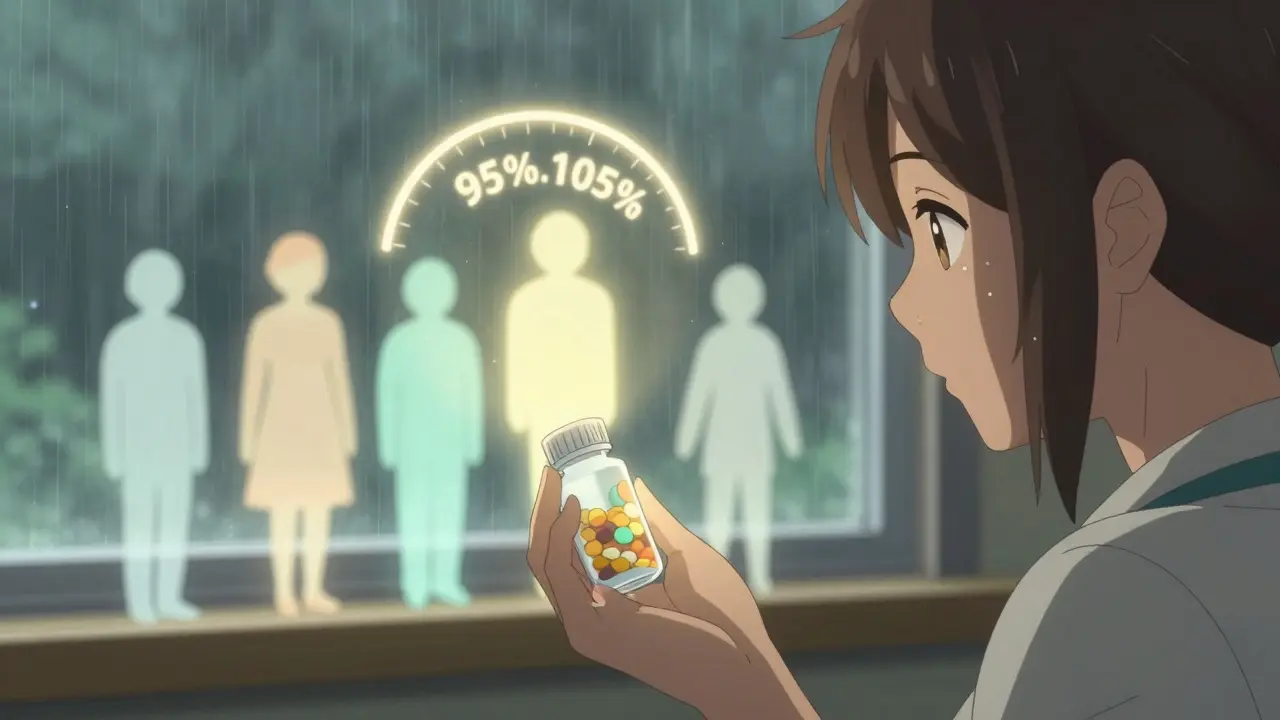
Are Generics Safe? The Evidence
Some patients worry that generics aren’t as safe. But the data says otherwise. A 2021 report from the American Medical Association reviewed 15 years of post-market data across 98.7% of therapeutic categories. They found no clinically meaningful differences in outcomes between generics and brand-name drugs. FDA Commissioner Robert Califf confirmed in 2023 that generics make up 90% of all prescriptions in the U.S. but only 23% of drug spending. That’s $373 billion in savings every year-money that keeps insulin, blood pressure meds, and antidepressants affordable. Dr. Janet Woodcock, former head of the FDA’s drug center, put it simply: “Every approved generic meets the same rigorous standards as the brand-name drug.”What’s Changing in 2025?
The FDA is pushing harder to get complex generics approved faster. Their goal? To approve 50% of complex generic applications within two review cycles by 2027. Right now, it’s only 28%. They’re also expanding their product-specific guidance. As of October 2023, they’ve published detailed requirements for over 2,800 brand-name drugs. That means manufacturers know exactly what data to submit-reducing delays. The first generic of Humira (adalimumab) was approved in December 2023 after years of delays. It’s a milestone-not just because it’s a blockbuster drug, but because it proved that even the most complex biologic-like drugs can be replicated with precision.What Should You Do?
If you’re prescribed a generic drug, you can trust it. The FDA’s system is designed to catch any deviation before it reaches your medicine cabinet. If you notice a change in how you feel after switching generics-like new side effects or reduced effectiveness-talk to your doctor. It’s not common, but it can happen, especially with narrow therapeutic index drugs. Don’t avoid generics because you think they’re “lesser.” They’re the reason millions of Americans can afford their prescriptions. And they’re held to the same standards as the originals.Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence-meaning they’re absorbed into your bloodstream at the same rate and to the same extent. Studies show no meaningful difference in effectiveness for 98.7% of therapeutic categories.
Why do some people say generics don’t work as well?
Sometimes, it’s not the drug-it’s the inactive ingredients. Fillers, dyes, or coatings can affect how quickly a pill dissolves or how your stomach reacts. For most people, this causes no issue. But with narrow therapeutic index drugs like warfarin or levothyroxine, even small changes in absorption can cause symptoms. If you notice a difference after switching, talk to your doctor about sticking with one version.
How does the FDA ensure generic drugs are made safely?
All manufacturing facilities must follow Current Good Manufacturing Practices (cGMP). The FDA conducts about 1,200 pre-approval inspections each year. They check everything from equipment cleanliness to batch consistency. If a facility has major deficiencies, approval is delayed until fixes are verified. For example, inconsistent tablet hardness once caused a complete rejection of a generic Jardiance application.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs-like pills with one active ingredient. Biosimilars are highly similar versions of complex biological drugs made from living cells, like Humira or insulin. Biosimilars require more testing because they’re harder to replicate exactly. The FDA treats them as a separate category with stricter requirements.
Why do some generic drugs take so long to get approved?
Even though the approval process is abbreviated, it’s not easy. Complex products like inhalers, injectables, or extended-release tablets require advanced testing and manufacturing control. Many applications are rejected on the first try because of incomplete data, manufacturing flaws, or patent issues. The average approval time is over 2.5 years, and complex generics can take nearly four years.

